 |
WARNINGTHE CLINICAL TOXICITY OF VALCYTE, WHICH IS METABOLIZED TO GANCICLOVIR, INCLUDES GRANULOCYTOPENIA, ANEMIA AND THROMBOCYTOPENIA. IN ANIMAL STUDIES GANCICLOVIR WAS CARCINOGENIC, TERATOGENIC AND CAUSED ASPERMATOGENESIS. |
Valcyte (valganciclovir HCl tablets) contains valganciclovir hydrochloride (valganciclovir HCl), a hydrochloride salt of the L-valyl ester of ganciclovir that exists as a mixture of two diastereomers. Ganciclovir is a synthetic guanine derivative active against cytomegalovirus (CMV).
Valganciclovir is available as a 450 mg tablet for oral administration. Each tablet contains 496.3 mg of valganciclovir HCl (corresponding to 450 mg of valganciclovir), and the inactive ingredients microcrystalline cellulose, povidone K-30, crospovidone, and stearic acid. The film-coat applied to the tablets contains Opadry Pink®.
Valganciclovir HCl is a white to off-white crystalline powder with a molecular formula of C 14 H 22 N 6 O 5 ·HCl and a molecular weight of 390.83. The chemical name for valganciclovir HCl is L-Valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9- yl)methoxy]-3-hydroxypropyl ester, monohydrochloride. Valganciclovir HCl is a polar hydrophilic compound with a solubility of 70 mg/mL in water at 25°C at a pH of 7.0 and an n-octanol/water partition coefficient of 0.0095 at pH 7.0. The pKa for valganciclovir is 7.6.
The chemical structure of valganciclovir HCl is:
 |
All doses in this insert are specified in terms of valganciclovir.
Valganciclovir is an L-valyl ester (prodrug) of ganciclovir that exists as a mixture of two diastereomers. After oral administration, both diastereomers are rapidly converted to ganciclovir by intestinal and hepatic esterases. Ganciclovir is a synthetic analogue of 2'-deoxyguanosine, which inhibits replication of human cytomegalovirus in vitro and in vivo.
In CMV-infected cells ganciclovir is initially phosphorylated to ganciclovir monophosphate by the viral protein kinase, pUL97. Further phosphorylation occurs by cellular kinases to produce ganciclovir triphosphate, which is then slowly metabolized intracellularly (half-life 18 hours). As the phosphorylation is largely dependent on the viral kinase, phosphorylation of ganciclovir occurs preferentially in virus-infected cells. The virustatic activity of ganciclovir is due to inhibition of viral DNA synthesis by ganciclovir triphosphate.
The quantitative relationship between the in vitro susceptibility of human herpesviruses to antivirals and clinical response to antiviral therapy has not been established, and virus sensitivity testing has not been standardized. Sensitivity test results, expressed as the concentration of drug required to inhibit the growth of virus in cell culture by 50% (IC 50 ), vary greatly depending upon a number of factors. Thus the IC 50 of ganciclovir that inhibits human CMV replication in vitro (laboratory and clinical isolates) has ranged from 0.02 to 5.75 µg/mL (0.08 to 22.94 µM). Ganciclovir inhibits mammalian cell proliferation (CIC 50 ) in vitro at higher concentrations ranging from 10.21 to >250 µg/mL (40 to >1000 µM). Bone marrow-derived colony-forming cells are more sensitive (CIC 50 = 0.69 to 3.06 µg/mL: 2.7 to 12 µM).
Viruses resistant to ganciclovir can arise after prolonged treatment with valganciclovir by selection of mutations in either the viral protein kinase gene (UL97) responsible for ganciclovir monophosphorylation and/or in the viral polymerase gene (UL54). Virus with mutations in the UL97 gene is resistant to ganciclovir alone, whereas virus with mutations in the UL54 gene may show cross-resistance to other antivirals with a similar mechanism of action.
The current working definition of CMV resistance to ganciclovir in in vitro assays is IC 50 >/=1.5 µg/mL (>/=6.0 µM). CMV resistance to ganciclovir has been observed in individuals with AIDS and CMV retinitis who have never received ganciclovir therapy. Viral resistance has also been observed in patients receiving prolonged treatment for CMV retinitis with ganciclovir. The possibility of viral resistance should be considered in patients who show poor clinical response or experience persistent viral excretion during therapy.
BECAUSE THE MAJOR ELIMINATION PATHWAY FOR GANCICLOVIR IS RENAL, DOSAGE REDUCTIONS ACCORDING TO CREATININE CLEARANCE ARE REQUIRED FOR VALCYTE TABLETS. FOR DOSING INSTRUCTIONS IN PATIENTS WITH RENAL IMPAIRMENT, REFER TO DOSAGE AND ADMINISTRATION .
The ganciclovir pharmacokinetic measures following administration of 900 mg valganciclovir and 5 mg/kg intravenous ganciclovir and 1000 mg three times daily oral ganciclovir are summarized in Table 1.
|
||||||||||||||||||||||||||||||||
The area under the plasma concentration-time curve (AUC) for ganciclovir administered as Valcyte tablets is comparable to the ganciclovir AUC for intravenous ganciclovir. Ganciclovir C max following valganciclovir administration is 40% lower than following intravenous ganciclovir administration. During maintenance dosing, ganciclovir AUC 0-24 hr and C max following oral ganciclovir administration (1000 mg three times daily) are lower relative to valganciclovir and intravenous ganciclovir. The ganciclovir C min following intravenous ganciclovir and valganciclovir administration are less than the ganciclovir C min following oral ganciclovir administration. The clinical significance of the differences in ganciclovir pharmacokinetics for these three ganciclovir delivery systems is unknown.
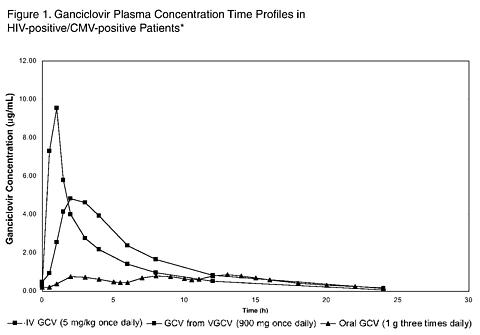 |
* Plasma concentration-time profiles for ganciclovir (GCV) from valganciclovir (VGCV) and intravenous ganciclovir were obtained from a multiple dose study (WV15376 n=21 and n=18, respectively) in HIV-positive/CMV-positive patients with CMV retinitis. The plasma concentration-time profile for oral ganciclovir was obtained from a multiple dose study (GAN2230 n=24) in HIV-positive/CMV-positive patients without CMV retinitis.
Valganciclovir, a prodrug of ganciclovir, is well absorbed from the gastrointestinal tract and rapidly metabolized in the intestinal wall and liver to ganciclovir. The absolute bioavailability of ganciclovir from Valcyte tablets following administration with food was approximately 60% (3 studies, n=18; n=16; n=28). Ganciclovir median T max following administration of 450 mg to 2625 mg valganciclovir tablets ranged from 1 to 3 hours. Dose proportionality with respect to ganciclovir AUC following administration of valganciclovir tablets was demonstrated only under fed conditions. Systemic exposure to the prodrug, valganciclovir, is transient and low, and the AUC 24 and C max values are approximately 1% and 3% of those of ganciclovir, respectively.
When valganciclovir tablets were administered with a high fat meal containing approximately 600 total calories (31.1 g fat, 51.6 g carbohydrates, and 22.2 g protein) at a dose of 875 mg once daily to 16 HIV-positive subjects, the steady-state ganciclovir AUC increased by 30% (95% CI 12-51%), and the C max increased by 14% (95% CI -5-36%), without any prolongation in time to peak plasma concentrations (T max ). Valcyte tablets should be administered with food (see DOSAGE AND ADMINISTRATION ).
Due to the rapid conversion of valganciclovir to ganciclovir, plasma protein binding of valganciclovir was not determined. Plasma protein binding of ganciclovir is 1% to 2% over concentrations of 0.5 and 51 µg/ml. When ganciclovir was administered intravenously, the steady state volume of distribution of ganciclovir was 0.703 ± 0.134 L/kg (n=69).
After administration of valganciclovir tablets, no correlation was observed between ganciclovir AUC and reciprocal weight; oral dosing of valganciclovir tablets according to weight is not required.
Valganciclovir is rapidly hydrolyzed to ganciclovir; no other metabolites have been detected. No metabolite of orally-administered radiolabeled ganciclovir (1000 mg single dose) accounted for more than 1% to 2% of the radioactivity recovered in the feces or urine.
The major route of elimination of valganciclovir is by renal excretion as ganciclovir through glomerular filtration and active tubular secretion. Systemic clearance of intravenously administered ganciclovir was 3.07 ± 0.64 mL/min/kg (n=68) while renal clearance was 2.99± 0.67 mL/min/kg (n=16).
The terminal half-life (t 1/2 ) of ganciclovir following oral administration of valganciclovir tablets to either healthy or HIV-positive/CMV-positive subjects was 4.08 ± 0.76 hours (n=73), and that following administration of intravenous ganciclovir was 3.81 ± 0.71 hours (n=69).
The pharmacokinetics of ganciclovir from a single oral dose of 900 mg Valcyte tablets were evaluated in 24 otherwise healthy individuals with renal impairment.
|
Decreased renal function results in decreased clearance of ganciclovir from valganciclovir, and a corresponding increase in terminal half-life. Therefore, dosage adjustment is required for patients with impaired renal function (see PRECAUTIONS : General ).
Hemodialysis reduces plasma concentrations of ganciclovir by about 50% following valganciclovir administration. Patients receiving hemodialysis (CrCl <10 ml/min) cannot use Valcyte tablets because the daily dose of Valcyte tablets required for these patients is less than 450 mg (see PRECAUTIONS : General and DOSAGE AND ADMINISTRATION : Hemodialysis Patients ).
Liver Transplant Patients
In liver transplant patients, the ganciclovir AUC 0-24 hr achieved with 900 mg valganciclovir was 41.7 ± 9.9 µg·h/mL (n=28) and the AUC 0-24 hr achieved with the approved dosage of 5 mg/kg intravenous ganciclovir was 48.2 ± 17.3 µg·h/mL (n=27).
Race/Ethnicity and Gender
Insufficient data are available to demonstrate any effect of race or gender on the pharmacokinetics of valganciclovir.
Valcyte tablets have not been studied in pediatric patients; the pharmacokinetic characteristics of Valcyte tablets in these patients have not been established (see PRECAUTIONS : Pediatric Use ).
Geriatrics
No studies of Valcyte tablets have been conducted in adults older than 65 years of age (see PRECAUTIONS : Geriatric Use ).
Valcyte tablets are indicated for the treatment of cytomegalovirus (CMV) retinitis in patients with acquired immunodeficiency syndrome (AIDS) (see CLINICAL TRIALS ).
In a randomized, open-label controlled study, 160 patients with AIDS and newly diagnosed CMV retinitis were randomized to receive treatment with either Valcyte tablets (900 mg twice daily for 21 days, then 900 mg once daily for 7 days) or with intravenous ganciclovir solution (5 mg/kg twice daily for 21 days, then 5 mg/kg once daily for 7 days). Study participants were: male (91%), White (53%), Hispanic (31%), and Black (11%). The median age was 39 years, the median baseline HIV-1 RNA was 4.9 log 10 , and the median CD4 cell count was 23 cells/mm 3 . A determination of CMV retinitis progression by the masked review of retinal photographs taken at baseline and week 4 was the primary outcome measurement of the three week induction therapy. Table 3 provides the outcomes at four weeks.
|
No comparative clinical data are available on the efficacy of Valcyte for the maintenance therapy of CMV retinitis because all patients in study WV15376 received open-label Valcyte after week 4. However, the AUC for ganciclovir is similar following administration of 900 mg valganciclovir once daily and 5 mg/kg intravenous ganciclovir once daily. Although the ganciclovir C max is lower following valganciclovir administration compared to intravenous ganciclovir, it is higher than the C max obtained following oral ganciclovir administration (see Figure 1 in CLINICAL PHARMACOLOGY ). Therefore, use of valganciclovir as maintenance therapy is supported by a plasma concentration-time profile similar to that of two approved products for maintenance therapy of CMV retinitis.
Valcyte tablets are contraindicated in patients with hypersensitivity to valganciclovir or ganciclovir.
THE CLINICAL TOXICITY OF VALCYTE, WHICH IS METABOLIZED TO GANCICLOVIR, INCLUDES GRANULOCYTOPENIA, ANEMIA AND THROMBOCYTOPENIA. IN ANIMAL STUDIES GANCICLOVIR WAS CARCINOGENIC, TERATOGENIC AND CAUSED ASPERMATOGENESIS.
Valcyte tablets should not be administered if the absolute neutrophil count is less than 500 cells/µL, the platelet count is less than 25,000/µL, or the hemoglobin is less than 8 g/dL.
Severe leukopenia, neutropenia, anemia, thrombocytopenia, pancytopenia, bone marrow depression and aplastic anemia have been observed in patients treated with Valcyte tablets (and ganciclovir) (see PRECAUTIONS : Laboratory Testing and ADVERSE EVENTS ).
Valcyte tablets should, therefore, be used with caution in patients with pre-existing cytopenias, or who have received or who are receiving myelosuppressive drugs or irradiation. Cytopenia may occur at any time during treatment and may increase with continued dosing. Cell counts usually begin to recover within 3 to 7 days of discontinuing drug.
Animal data indicate that administration of ganciclovir causes inhibition of spermatogenesis and subsequent infertility. These effects were reversible at lower doses and irreversible at higher doses (see PRECAUTIONS : Carcinogenesis, Mutagenesis and Impairment of Fertility ). It is considered probable that in humans, valganciclovir at the recommended doses may cause temporary or permanent inhibition of spermatogenesis. Animal data also indicate that suppression of fertility in females may occur.
Because of the mutagenic and teratogenic potential of ganciclovir, women of childbearing potential should be advised to use effective contraception during treatment. Similarly, men should be advised to practice barrier contraception during, and for at least 90 days following, treatment with Valcyte tablets (see PRECAUTIONS : Carcinogenesis, Mutagenesis and Pregnancy: Category C ).
In animal studies, ganciclovir was found to be mutagenic and carcinogenic. Valganciclovir should, therefore, be considered a potential teratogen and carcinogen in humans with the potential to cause birth defects and cancers (see DOSAGE AND ADMINISTRATION : Handling and Disposal ).
Strict adherence to dosage recommendations is essential to avoid overdose.
The bioavailability of ganciclovir from Valcyte tablets is significantly higher than from ganciclovir capsules. Patients switching from ganciclovir capsules should be advised of the risk of overdosage if they take more than the prescribed number of Valcyte tablets. Valcyte tablets cannot be substituted for Cytovene capsules on a one-to-one basis (see OVERDOSAGE and DOSAGE AND ADMINISTRATION ).
Since ganciclovir is excreted by the kidneys, normal clearance depends on adequate renal function. IF RENAL FUNCTION IS IMPAIRED, DOSAGE ADJUSTMENTS ARE REQUIRED FOR VALCYTE TABLETS. Such adjustments should be based on measured or estimated creatinine clearance values (see DOSAGE AND ADMINISTRATION : Renal Impairment ).
For patients on hemodialysis (CrCl <10 mL/min) it is recommended that ganciclovir be used (in accordance with the dose-reduction algorithm cited in the Cytovene®-IV and Cytovene® Package Insert section on DOSAGE AND ADMINISTRATION : Renal Impairment ) rather than Valcyte tablets (see DOSAGE AND ADMINISTRATION : Hemodialysis and CLINICAL PHARMACOLOGY : Special Populations : Hemodialysis ).
Valcyte tablets cannot be substituted for ganciclovir capsules on a one-to-one basis. Patients switching from ganciclovir capsules should be advised of the risk of overdosage if they take more than the prescribed number of Valcyte tablets (see OVERDOSAGE and DOSAGE AND ADMINISTRATION ).
Valcyte is changed to ganciclovir once it is absorbed into the body. All patients should be informed that the major toxicities of ganciclovir include granulocytopenia (neutropenia), anemia and thrombocytopenia and that dose modifications may be required, including discontinuation. The importance of close monitoring of blood counts while on therapy should be emphasized. Patients should be informed that ganciclovir has been associated with elevations in serum creatinine.
Patients should be instructed to take Valcyte tablets with food to maximize bioavailability.
Patients should be advised that ganciclovir has caused decreased sperm production in animals and may cause decreased fertility in humans. Women of childbearing potential should be advised that ganciclovir causes birth defects in animals and should not be used during pregnancy. Because of the potential for serious adverse events in nursing infants, mothers should be instructed not to breastfeed if they are receiving Valcyte tablets. Women of childbearing potential should be advised to use effective contraception during treatment with Valcyte tablets. Similarly, men should be advised to practice barrier contraception during and for at least 90 days following treatment with Valcyte tablets.
Although there is no information from human studies, patients should be advised that ganciclovir should be considered a potential carcinogen.
Convulsions, sedation, dizziness, ataxia and/or confusion have been reported with the use of Valcyte tablets and/or ganciclovir. If they occur, such effects may affect tasks requiring alertness including the patient' ability to drive and operate machinery.
Patients should be told that ganciclovir is not a cure for CMV retinitis, and that they may continue to experience progression of retinitis during or following treatment. Patients should be advised to have ophthalmologic follow-up examinations at a minimum of every 4 to 6 weeks while being treated with Valcyte tablets. Some patients will require more frequent follow-up.
Due to the frequency of neutropenia, anemia and thrombocytopenia in patients receiving Valcyte tablets (see ADVERSE EVENTS ), it is recommended that complete blood counts and platelet counts be performed frequently, especially in patients in whom ganciclovir or other nucleoside analogues have previously resulted in leukopenia, or in whom neutrophil counts are less than 1000 cells/µL at the beginning of treatment. Increased monitoring for cytopenias may be warranted if therapy with oral ganciclovir is changed to oral valganciclovir, because of increased plasma concentrations of ganciclovir after valganciclovir administration (see CLINICAL PHARMACOLOGY ).
Increased serum creatinine levels have been observed in trials evaluating Valcyte tablets. Patients should have serum creatinine or creatinine clearance values monitored carefully to allow for dosage adjustments in renally impaired patients (see DOSAGE AND ADMINISTRATION : Renal Impairment ). The mechanism of impairment of renal function is not known.
No in vivo drug-drug interaction studies were conducted with valganciclovir. However, because valganciclovir is rapidly and extensively converted to ganciclovir, interactions associated with ganciclovir will be expected for Valcyte tablets.
Binding of ganciclovir to plasma proteins is only about 1% to 2%, and drug interactions involving binding site displacement are not anticipated.
Drug-drug interaction studies were conducted in patients with normal renal function. Patients with impaired renal function may have increased concentrations of ganciclovir and the coadministered drug following concomitant administration of Valcyte tablets and drugs excreted by the same pathway as ganciclovir. Therefore, these patients should be closely monitored for toxicity of ganciclovir and the coadministered drug.
|
|
No long-term carcinogenicity studies have been conducted with valganciclovir. However, upon oral administration, valganciclovir is rapidly and extensively converted to ganciclovir. Therefore, like ganciclovir, valganciclovir is a potential carcinogen.
Ganciclovir was carcinogenic in the mouse at oral doses of 20 and 1000 mg/kg/day (approximately 0.1 × and 1.4 × , respectively, the mean drug exposure in humans following the recommended intravenous dose of 5 mg/kg, based on area under the plasma concentration curve [AUC] comparisons). At the dose of 1000 mg/kg/day there was a significant increase in the incidence of tumors of the preputial gland in males, forestomach (nonglandular mucosa) in males and females, and reproductive tissues (ovaries, uterus, mammary gland, clitoral gland and vagina) and liver in females. At the dose of 20 mg/kg/day, a slightly increased incidence of tumors was noted in the preputial and harderian glands in males, forestomach in males and females, and liver in females. No carcinogenic effect was observed in mice administered ganciclovir at 1 mg/kg/day (estimated as 0.01 × the human dose based on AUC comparison). Ganciclovir should be considered a potential carcinogen in humans.
Valganciclovir increases mutations in mouse lymphoma cells. In the mouse micronucleus assay, valganciclovir was clastogenic at a dose of 1500 mg/kg (60 × human mean exposure for ganciclovir based upon AUC). Valganciclovir was not mutagenic in the Ames Salmonella assay. Ganciclovir increased mutations in mouse lymphoma cells and DNA damage in human lymphocytes in vitro. In the mouse micronucleus assay, ganciclovir was clastogenic at doses of 150 and 500 mg/kg (IV) (2.8 to 10x human exposure based on AUC) but not 50 mg/kg (exposure approximately comparable to the human based on AUC). Ganciclovir was not mutagenic in the Ames Salmonella assay.
Valganciclovir is converted to ganciclovir and therefore is expected to have similar reproductive toxicity effects as ganciclovir (see WARNINGS : Impairment of Fertility ). Ganciclovir caused decreased mating behavior, decreased fertility, and an increased incidence of embryolethality in female mice following intravenous doses of 90 mg/kg/day (approximately 1.7 × the mean drug exposure in humans following the dose of 5 mg/kg, based on AUC comparisons). Ganciclovir caused decreased fertility in male mice and hypospermatogenesis in mice and dogs following daily oral or intravenous administration of doses ranging from 0.2 to 10 mg/kg. Systemic drug exposure (AUC) at the lowest dose showing toxicity in each species ranged from 0.03 to 0.1 × the AUC of the recommended human intravenous dose. Valganciclovir caused similar effects on spermatogenesis in mice, rats, and dogs. It is considered likely that ganciclovir (and valganciclovir) could cause inhibition of human spermatogenesis.
Valganciclovir is converted to ganciclovir and therefore is expected to have reproductive toxicity effects similar to ganciclovir. Ganciclovir has been shown to be embryotoxic in rabbits and mice following intravenous administration, and teratogenic in rabbits. Fetal resorptions were present in at least 85% of rabbits and mice administered 60 mg/kg/day and 108 mg/kg/day (2 × the human exposure based on AUC comparisons), respectively. Effects observed in rabbits included: fetal growth retardation, embryolethality, teratogenicity and/or maternal toxicity. Teratogenic changes included cleft palate, anophthalmia/microphthalmia, aplastic organs (kidney and pancreas), hydrocephaly and brachygnathia. In mice, effects observed were maternal/fetal toxicity and embryolethality.
Daily intravenous doses of 90 mg/kg administered to female mice prior to mating, during gestation, and during lactation caused hypoplasia of the testes and seminal vesicles in the month-old male offspring, as well as pathologic changes in the nonglandular region of the stomach (see Teratogenesis, Carcinogenesis and Mutagenesis ). The drug exposure in mice as estimated by the AUC was approximately 1.7 × the human AUC.
Data obtained using an ex vivo human placental model show that ganciclovir crosses the placenta and that simple diffusion is the most likely mechanism of transfer. The transfer was not saturable over a concentration range of 1 to 10 mg/mL and occurred by passive diffusion.
Valganciclovir may be teratogenic or embryotoxic at dose levels recommended for human use. There are no adequate and well-controlled studies in pregnant women. Valcyte tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
** Footnote: All dose comparisons presented in the Carcinogenesis, Mutagenesis, Impairment of Fertility, and Pregnancy subsections are based on the human AUC following administration of a single 5 mg/kg infusion of intravenous ganciclovir.
It is not known whether ganciclovir or valganciclovir is excreted in human milk. Because valganciclovir caused granulocytopenia, anemia and thrombocytopenia in clinical trials and ganciclovir was mutagenic and carcinogenic in animal studies, the possibility of serious adverse events from ganciclovir in nursing infants is possible (see WARNINGS ). Because of potential for serious adverse events in nursing infants, mothers should be instructed not to breastfeed if they are receiving Valcyte tablets. In addition, the Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV.
Safety and effectiveness of Valcyte tablets in pediatric patients have not been established.
The pharmacokinetic characteristics of Valcyte in elderly patients have not been established. Since elderly individuals frequently have a reduced glomerular filtration rate, particular attention should be paid to assessing renal function before and during administration of Valcyte (see DOSAGE AND ADMINISTRATION ).
Clinical studies of Valcyte did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Valcyte is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection. In addition, renal function should be monitored and dosage adjustments should be made accordingly (see PRECAUTIONS : General , CLINICAL PHARMACOLOGY : Special Populations : Renal Impairment , and DOSAGE AND ADMINISTRATION : Renal Impairment ).
Valganciclovir, a prodrug of ganciclovir, is rapidly converted to ganciclovir after oral administration. Adverse events known to be associated with ganciclovir usage can therefore be expected to occur with Valcyte tablets.
As shown in Table 6, the safety profiles of Valcyte tablets and intravenous ganciclovir during 28 days of randomized therapy (21 days induction dose and 7 days maintenance dose) in 158 patients were comparable, with the exception of catheter-related infection, which occurred with greater frequency in patients randomized to receive IV ganciclovir (see Table 6).
|
Tables 7 and 8 show the pooled adverse event data and abnormal laboratory values from two single arm, open-label clinical trials, WV15376 (after the initial four weeks of randomized therapy) and WV15705. A total of 370 patients received maintenance therapy with valganciclovir tablets 900 mg q day. Approximately 252 (68%) of these patients received Valcyte tablets for more than nine months (maximum duration was 36 months).
|
||||||||||||||||||||||||||||||||||||||
Serious adverse events reported from these two clinical trials (N=370) with a frequency of less than 5% and which are not mentioned in the two tables above, are listed below:
Hemic and lymphatic system: pancytopenia, bone marrow depression, aplastic anemia
Urogenital system: decreased creatinine clearance
Infections: local and systemic infections and sepsis
Bleeding complications: potentially life-threatening bleeding associated with thrombocytopenia
Central and peripheral nervous system: convulsion, psychosis, hallucinations, confusion, agitation
Body as a whole: valganciclovir hypersensitivity
Laboratory abnormalities reported with Valcyte tablets are listed below:
|
||||||||||||||||||||||||||||||||
Valganciclovir is rapidly converted to ganciclovir upon oral administration. Adverse events reported with Valcyte in general were similar to those reported with ganciclovir (Cytovene). Please refer to the Cytovene label for more information on post-marketing adverse events associated with ganciclovir.
One adult developed fatal bone marrow depression (medullary aplasia) after several days of dosing that was at least 10-fold greater than recommended for the patient' estimated degree of renal impairment.
It is expected that an overdose of Valcyte tablets could also possibly result in increased renal toxicity (see PRECAUTIONS : General and DOSAGE AND ADMINISTRATION : Renal Impairment ).
Since ganciclovir is dialyzable, dialysis may be useful in reducing serum concentrations in patients who have received an overdose of Valcyte tablets (see CLINICAL PHARMACOLOGY : Special Populations : Hemodialysis ). Adequate hydration should be maintained. The use of hematopoietic growth factors should be considered (see CLINICAL PHARMACOLOGY: Special Populations : Hemodialysis ).
Reports of overdoses with intravenous ganciclovir have been received from clinical trials and during postmarketing experience. The majority of patients experienced one or more of the following adverse events:
Hematological toxicity: pancytopenia, bone marrow depression, medullary aplasia, leukopenia, neutropenia, granulocytopenia
Hepatotoxicity: hepatitis, liver function disorder
Renal toxicity: worsening of hematuria in a patient with pre-existing renal impairment, acute renal failure, elevated creatinine
Gastrointestinal toxicity: abdominal pain, diarrhea, vomiting
Neurotoxicity: generalized tremor, convulsion
Strict adherence to dosage recommendations is essential to avoid overdose. Valcyte tablets cannot be substituted for Cytovene capsules on a one-to-one basis.
Valcyte tablets are administered orally, and should be taken with food (see CLINICAL PHARMACOLOGY : Absorption ). After oral administration, valganciclovir is rapidly and extensively converted into ganciclovir. The bioavailability of ganciclovir from Valcyte tablets is significantly higher than from ganciclovir capsules. Therefore the dosage and administration of Valcyte tablets as described below should be closely followed (see PRECAUTIONS : General and OVERDOSAGE ).
|
Induction:
For patients with active CMV retinitis, the recommended dosage is 900 mg (two 450 mg tablets)
Maintenance:
Following induction treatment, or in patients with inactive CMV retinitis, the recommended dosage
|
Serum creatinine or creatinine clearance levels should be monitored carefully. Dosage adjustment is required according to creatinine clearance as shown in the table below (see PRECAUTIONS : General and CLINICAL PHARMACOLOGY : Special Populations : Renal Impairment ). Increased monitoring for cytopenias may be warranted in patients with renal impairment (see PRECAUTIONS : Laboratory Testing ).
|
||||||||||||||||||
Valcyte should not be prescribed to patients receiving hemodialysis (see CLINICAL PHARMACOLOGY : Special Populations : Hemodialysis and PRECAUTIONS : General ).
Caution should be exercised in the handling of Valcyte tablets. Tablets should not be broken or crushed. Since valganciclovir is considered a potential teratogen and carcinogen in humans, caution should be observed in handling broken tablets (see WARNINGS : Teratogenesis, Carcinogenesis and Mutagenesis ). Avoid direct contact of broken or crushed tablets with skin or mucous membranes. If such contact occurs, wash thoroughly with soap and water, and rinse eyes thoroughly with plain water.
Because ganciclovir shares some of the properties of antitumor agents (ie, carcinogenicity and mutagenicity), consideration should be given to handling and disposal according to guidelines issued for antineoplastic drugs. Several guidelines on this subject have been published (see REFERENCES ).
There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
Valcyte (valganciclovir HCl tablets) is available as 450 mg pink convex oval tablets with "VGC" on one side and "450" on the other side. Each tablet contains valganciclovir HCl equivalent to 450 mg valganciclovir. Valcyte is supplied in bottles of 60 tablets (NDC 0004-0038-22).
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP controlled room temperature].
Cytovene is a registered trademark of Syntex (U.S.A.) LLC.
Valcyte tablets are manufactured by Patheon Inc., Mississauga, Ontario, Canada L5N 7K9
Rx only
Distributed by:
Roche Pharmaceuticals
Roche Laboratories Inc.
340 Kingsland Street
Nutley, New Jersey 07110-1199
Issued: March 2001
Copyright © 2001 by Roche Laboratories Inc. All rights reserved.